COVALENT BONDING IN CARBON COMPOUNDS
Covalent bonds are formed when two atoms (either identical or dissimilar) share an electron pair.
Carbon (C) is a six-atomic element.
So, its electronic configuration = K/2, L/4
Thus, there are 4 electrons in its outermost shell and its octet can be completed by the following two ways
- It is possible for it to gain four electrons and form the C4⁻ anion. However, for a nucleus with six protons, it would be difficult to retain ten electrons, i.e. four additional electrons.
- It is possible for it to lose four electrons and form the C4⁺ cation. However, removing four electrons would require an enormous amount of energy, leaving behind a carbon cation with six protons in its nucleus and only two electrons, which is not possible.
To solve this issue, carbon shares its valence electrons with other carbon atoms or with other elements. These shared electrons are located in both atoms’ outermost shells, and as a result, both atoms attain the nearest noble gas configuration. This is referred to as covalent bonding.
Covalent compounds are those that contain covalent bonds; they are generally poor conductors of electricity.
SOME EXAMPLES DEPICTING COVALENT BONDING
Some example depicting of covalent bonding are as follows
Formation of Hydrogen Molecule H₂
- Atomic number of H = 1
- Electronic configuration = K/1
- It has 1 electron in its K-shell and needs 1 more electron to fill the K-shell completely.
- Thus, two H-atoms share their electrons to form a molecule of Hydrogen This allows each H-atom to attain the nearest noble gas configuration, i.e. configuration of helium (having two electrons in its K -shell). Valence electrons are depicted by using crosses.
- The shared pair of electrons constitute a single bond between the two H-atoms, which is represented by a single line between the two atoms.
Formation of Chlorine Molecule CI₂
- Atomic number of Cl = 17
Electronic configuration = K/2, L/8, M/7 - It has 7 electrons in its outermost shell and thus require 1 more electron to fulfill its outermost shell. This is achieved by sharing 1 electron with another Cl-atom, forming a chlorine diatomic molecule (CI₂).

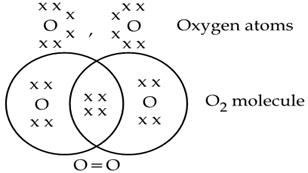
Formation of Oxygen Molecule O₂
- Atomic number of O = 8
- Electronic configuration = 2 , 6
- It has 6 electrons in its outermost shell thus, require 2 electrons to complete its octet for attaining noble gas configuration. This is achieved by sharing 2 electrons with another oxygen atom. The two electrons contributed by each oxygen atom give rise to two shared pairs of electrons.
Formation of Nitrogen Molecule N₂
- Atomic number of N = 7.
- Electronic configuration = K/2, L/5
- Nitrogen needs 3 more electrons to attain noble gas configuration. Thus, in order to attain octet each nitrogen atom in nitrogen molecule contributes three electrons giving rise to three shared pair of electrons.
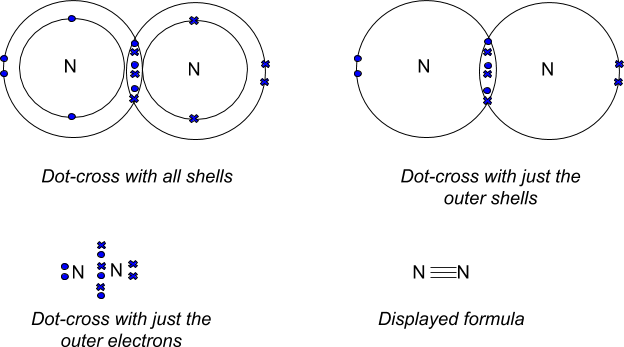
Here, a triple bond is formed between the two nitrogen atoms.
Formation of Methane CH₄
In the formation of a methane molecule, one carbon atom shares its 4 electrons with four hydrogen atoms (one electron of each hydrogen atom). It shows carbon is tetravalent because it possesses 4 valence electrons and hydrogen is monovalent because it has only 1 valence electron.

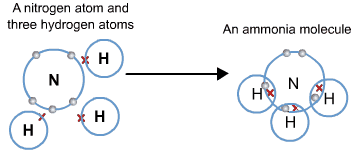
Formation of Ammonia NH₃ and Water Molecule H₂O Ammonia (NH₃)
- Atomic number of N = 7
- Electronic configuration = K/2, L/5
- Atomic number of H = 1
- Electronic configuration = K/1
- Nitrogen requires three electrons and hydrogen only one electron to achieve the electronic configuration of the nearest noble gas. When an ammonia molecule is formed, one nitrogen atom shares its three electrons with each of the three hydrogen atoms.
Water (H₂O)
- Atomic number of O = 8
- Electronic configuration = 2, 6
- Atomic number of H = 1
- Electronic configuration = K/1
- To attain the stable electronic configuration of the nearest noble gas, hydrogen needs 1 electron and oxygen needs 2 electrons.
- In case of a water molecule, two hydrogen atoms share an electron pair with the oxygen atom such that hydrogen acquires a duplet configuration and oxygen an octet, resulting in the formation of two single covalent bonds.

Formation of Carbon Dioxide CO₂
- Atomic number of C = 6
- Electronic configuration = K/2, L/4
- Atomic number of O = 8
- Electronic configuration = K/2, L/6
- To attain the stable electronic configuration, carbon needs 4 electrons, while oxygen needs 2 electrons. So, in CO₂, each of oxygen atom share two electrons from carbon. Thus, oxygen and carbon both attain octet.

Formation of Sulphur Molecule S₈
- Atomic number of sulphur (S) = 16
- Electronic configuration = K/2, L/8, M/6
- To attain the electronic configuration of the nearest noble gas, each sulphur needs 2 electrons.

PROPERTIES OF COVALENT COMPOUNDS
The compounds containing covalent bonds are called covalent compounds. They have following properties i.e.
- Covalent compounds have low melting and boiling points due to small intermolecular forces of attraction between the atoms.
- Covalent compounds are generally poor conductors of electricity. This is because the electrons are shared between atoms and no charged particles are formed in these compounds.
- Covalent compounds are generally volatile in nature.
Allotropes Of Carbon And Their Properties
Allotropy is the property by virtue of which an element exists in more than one form and each form has different physical properties but identical chemical properties. These different forms are called allotropes. Carbon exists in different allotropic forms; some of them are:
- Crystalline form, e.g. diamond, graphite and fullerene.
- Micro-crystalline form or amorphous form, e.g. coal, lampblack and charcoal.
Diamond
General Properties
- It is a colourless transparent substance with extra ordinary brilliance due to its high refractive index.
- It is quite heavy and extremely hard (hardest natural substance known).
- It does not conduct electricity (because of the absence of free electrons) but it has high thermal conductivity and high melting point.
Structure
It is a giant molecule of carbon atoms in which each carbon atom is bonded to four other carbon atoms forming a rigid three dimensional network structure, which is responsible for its hardness.
Moreover, a lot of energy is required break the network of strong covalent bonds in the diamond crystal. Therefore, its melting point is very high.
Graphite
General Properties
- It is a greyish black, opaque substance.
- It is lighter than diamond, smooth and slippery to touch.
- It is a good conductor of electricity (due to the presence of free electrons) but bad conductor of heat.
Structure
A graphite crystal consists of layers of carbon atoms or sheets of carbon atoms. Each carbon atom in a graphite layer is joined to three other carbon atoms by strong covalent bonds to form flat hexagonal rings.
However, the fourth electron of each carbon atom is free which makes it a good conductor of electricity. The various layers of carbon atoms in graphite are held together by weak van der Waals’ forces so these can slide over one another and therefore, graphite is slippery to touch.
Fullerenes
These are recently discovered allotropic forms of carbon which were prepared for the first time by H W Kroto, Smalley and Robert Curt by the action of laser beam on the vapours of graphite.
The first known fullerene was c₆₀ which contains 60 carbon atoms (c₆₀) with smaller proportion of (c₇₀) and traces of compounds containing even upto 3₇₀ carbon atoms.
Fullerene (c₆₀) was named Buckminster fullerene due to their resemblance (in structure) with geodesic domes, designed and built by the American Architect Robert Buckminster Fuller.
Versatile Nature Of Carbon
The estimated number of carbon compounds known today is about three million. But now the question is, which property or properties of carbon is/are responsible for the formation of such a large number of carbon compounds. Main factors that enables carbon to form large number of compound are:
- Catenation : The property of self linking of elements mainly C-atoms through covalent bonds to form long, straight or branched chains and rings of different sizes is called catenation. Carbon shows maximum catenation in the periodic table due to its small size and strong C – C bond. Hence, stable.
- Tetravalency of Carbon : Carbon belongs to group 14 of the periodic table. Its atomic number is 6 and the electronic configuration is 2, 4. Thus, it has four electrons in the outermost shell. Hence, its valency is four, i.e. it is capable of bonding or pairing with four ocher carbon atoms or with the atoms of some ocher monovalent elements like hydrogen, halogen (chlorine, bromine) etc.
- Tendency to form Multiple Bonds : Due to its small size carbon has a strong tendency to form multiple bonds (double and triple bonds) by sharing more than one electron pair with its own atoms or with the atoms of elements like oxygen, nitrogen, sulphur etc. As a result, it can form a variety of compounds that are exceptionally stable.
Organic Compounds
The compounds of carbon except its oxides, carbonates and hydrogen carbonate salts, are known as organic compounds. These compounds were initially extracted from natural substances and it was thought that these carbon compounds could only be formed within a living system. Thus, it was postulated that a ‘vital force’ was necessary for their synthesis.
In 1828, German chemist Friedrich Wohler accidently prepared urea from ammonium cyanate when he was trying to prepare ammonium cyanate by heating ammonium sulphate and potassium cyanate. Thus, synthesis of urea discarded the vital force theory.
Hydrocarbons
The organic compounds containing only carbon and hydrogen are called hydrocarbons. e.g. CH₄, C2H6, C₂H₄ and C2H2. These are the simplest organic compounds and are regarded as parent organic compounds.
All other compounds are considered to be derived from them by the replacement of one or more hydrogen atoms by other atoms or group of atoms. The hydrocarbons can be classified as:
- Saturated hydrocarbons
- Unsaturated hydrocarbons
Saturated Hydrocarbons
The hydrocarbons in which all the carbon atoms are linked by only single covalent bonds are called saturated hydrocarbons or alkanes or paraffins.
The general formula of these compounds is CnH₂n₊₂ ,
where, n = number of carbon atoms in one molecule of a hydrocarbon. e.g. if there is only one carbon atom then its formula should be C₁H₂ₓ₁₊₄ = CH₄ (methane).
Similarly, if there are two carbon atoms in the saturated hydrocarbon (alkane), its formula must be C₂H₂ₓ₂₊₂= C₂H₆ (ethane)
Unsaturated Hydrocarbons
The hydrocarbons in which atleast one double or triple bond (or multiple bond) is present alongwith single bonds are called unsaturated compounds.
These compounds generally burn with sooty or smoky flame due to their incomplete combustion. These are more reactive than saturated hydrocarbons and generally undergo addition reactions (which are discussed later in the chapter).
Unsaturated compounds are further divided into following two classes:
- Alkenes or Olefins : Those unsaturated hydrocarbons which have atleast one double bond alongwith single bonds are called alkenes. (A double bond is formed by sharing of two pairs of electrons between the two carbon atoms).General formula of these compounds is CnH₂n.
e.g. if an alkene have 2 carbon atoms, i.e. n = 2, its formula is C2H2ₓ₂=C₂H₄ (ethene). - Alkynes : Those unsaturated hydrocarbons which have one or more triple bonds alongwith the single bonds are called alkynes. (A triple bond is formed by the sharing of 3 pairs of electrons between two carbon atoms).
General formula of these compounds is CnH₂n-₂
e.g. if an alkyne have two carbon atoms, then its formula is C2H2ₓ₂-₂ = C2H2 (ethyne). If there are three carbon atoms in the alkyne then its formula must be C₃H₂ₓ₃-₂ = C₃H₄ (propyne).
How To Draw The Structure Of Saturated And Unsaturated Compounds?
Steps to draw the structure of carbon compound :
Step I. – First connect all the carbon atoms together with a single bond.
Step II. – After that use the hydrogen atoms to satisfy the remaining valencies of carbon (as carbon forms 4 bonds due to its 4 valency).
Step III. – If number of available H-atoms are less than what is required, satisfy the remaining valency by using double or triple bond.
Structure of Ethane C2H6
Step I. – In ethane, two carbon atoms are present. To find the structure of simple carbon compounds, the first step is to link the two C-atoms together with a single bond, i.e.
C-C
Step II. – Here, only 1 valency of carbon is satisfied and the 3 valencies of each carbon atom remain unsatisfied. Each of these unsatisfied valencies is satisfied by using H-atoms.

Now, the tetravalency of carbon in ethane is satisfied. Electron dot structure of ethane (C₂H₆).

Structure of Propane C₂H₈
Step I. – Same rules are followed here as in case of ethane. Here, the three carbon atoms are linked together with a single bond.
C-C-C
Step II. – To satisfy the remaining valencies of carbon atoms, hydrogen atoms are linked with them.
2 carbon atoms are bonded to 3 hydrogen atoms and 1 carbon atom is bonded to 2 hydrogen atoms.

Electron dot structure of propane

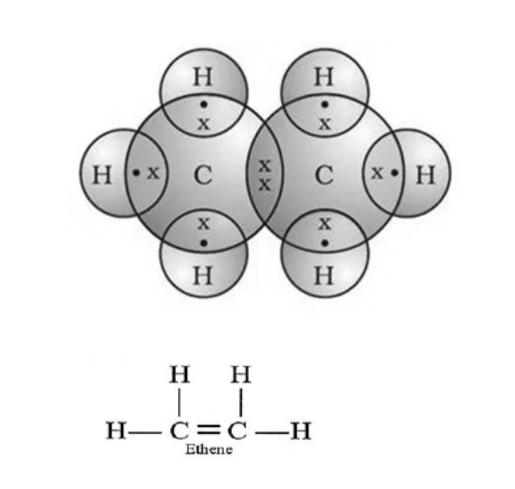
Structure of Ethene C₂H₄
Step I. – Link the two carbon atoms by single bond.
C-C
Step II. – Link the four hydrogen atoms with carbon atom to satisfy the unsatisfied valencies of carbon.
Step III. – But in this case, even after linking the available hydrogen atoms with carbon atoms, still one valency of each carbon remains unsatisfy.
To satisfy it, a double bond is used between the two carbon atoms.
Now, all the four valencies of carbon are satisfied.
Electron dot structure of ethene
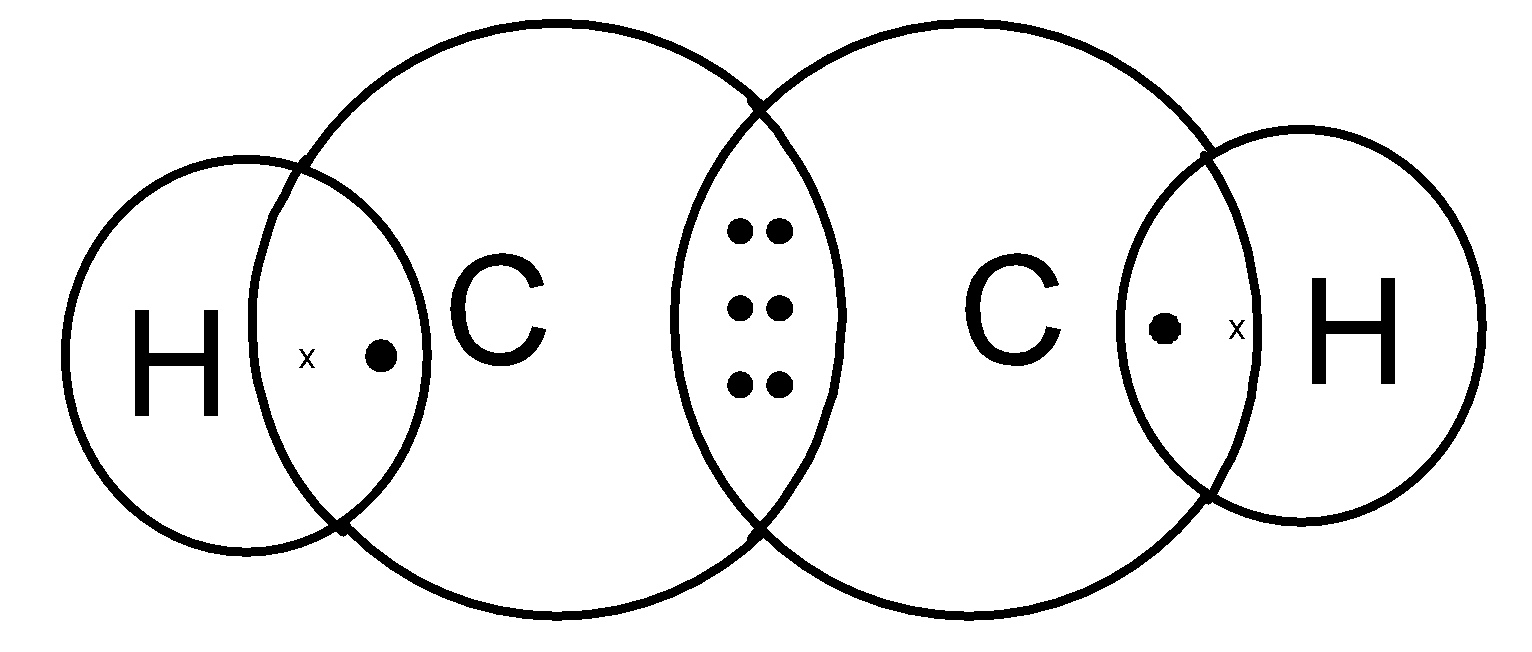
Structure of Ethyne C2H2
Step I. – Link the two carbon atoms by single bond.
C-C
Step II. – Link the two hydrogen atoms with unsatisfied valencies of carbon.
H-C-C-H
Step III. – But in this case even after linking the available hydrogen atoms with carbon atoms, still two valencies of each carbon is unsatisfy. To satisfy it, a triple bond is used between the two carbon atoms.
In ethyne, the two carbon atoms share three pairs of electrons among themselves to form a carbon-carbon triple bond.
Each carbon atom shares one electron with each hydrogen atom to form two carbon-hydrogen single bonds.
Electron dot structure of ethyne
How to Draw Structure of Cyclic Compounds?
Some carbon compounds also exist in cyclic or ring structure. To draw the structure of cyclic or ring compounds:
Step I. – First connect the available carbon atoms by a single bond in the cyclic form.
Step II. – Try to satisfy the tetravalency of each carbon with the available hydrogen atoms.
Step III. – Now check the valency of each carbon. If it is found unsatisfied, use double or triple bond to satisfy it.
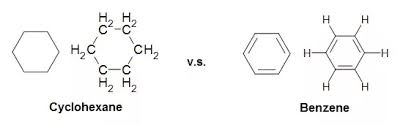
Cyclohexane (C₆h₁₂)
Step I. – Linking of carbon atoms by single bond.
Step II. – Linking of H-atoms with unsatisfied valencies of carbon.

As all the 4 valencies of each C-atom are satisfied. Therefore, there is no need to draw double or triple bond.
Benzene (C₆h₆)
Step I. – Linking of carbon atoms by single bond.
Step II. – Linking of hydrogen atoms with unsatisfied valencies of carbon.
But in this case, even after linking the available H-atoms with C-atoms, still one valency of each carbon remains unsatisfied. To satisfy it, a double bond is used between the two C-atoms.
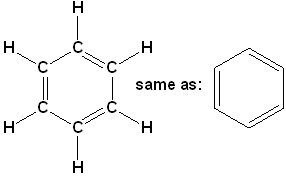
Now, all the four valencies of each C-atom are satisfied.
Isomerism
Organic compounds with same molecular formula but different chemical and physical properties are called isomers. This phenomenon is called isomerism.
The difference in properties of these compounds is due to the difference in their structures. These compounds have identical molecular formula but different structures. Hence, they are called structural isomers and phenomenon is called structural isomerism.
e.g. Two structural isomers are possible for butane (C₄H₁₀).

Similarly, in some compounds, carbon atoms are arranged in the form of ring. For example, cyclohexane C₄H₁₂ and benzene C₆H₆ .
Functional Groups
Carbon can also forms bonds with other elements such as halogens, oxygen, nitrogen, sulphur etc. These are called heteroatoms.
These atoms or the group of atoms, replace one or more hydrogen atoms of the hydrocarbon and are responsible for the chemical reactivity of the compound, regardless of the length and nature of carbon chain. Hence, these are called functional groups.
Thus, functional groups may be defined as an ‘atom’ or a ‘group of atoms’ which makes a carbon compound (or organic compound) reactive and decide its properties (or functions) regardless of the length and nature of carbon chain.
Homologous Series
A series of similarly constituted compounds in which the members present have the same functional group and similar chemical properties and any two successive members in a particular series differ in their molecular formula by a –CH₂– unit, is called a homologous series.
e.g. CH₄, C2H6, C3H8, C₄H₁₀ are the members of alkane family.
Characteristics Of A Homologous Series
- All the members of a homologous series can be represented by the same general formula.
- Any two adjacent homologues differ by 1 carbon atom and 2 hydrogen atoms in their molecular formula.
- All the compounds of a homologous series show similar chemical properties.
- With increase in the molecular mass, a gradual change in the physical properties is seen e.g. the melting and boiling points increase with increasing molecular mass.
- The difference in the molecular masses of any two adjacent homologues or members is 14 u.
Nomenclature Of Carbon Compounds
Generally, organic compounds have two names i.e. IUPAC and common names. The IUPAC names have been adopted by the International Union of Pure and Applied Chemistry and are based on certain rules.
The common names (also known as trivial names) have no proper system for naming. In general, IUPAC name of organic compounds are based on the name of parent carbon chain modified by a prefix (phrase before) or suffix (phrase after) indicating the name (or nature) of the functional group.
Writing Iupac Name Of A Compound
Following steps are used to write the name of an organic compound:
Step I. – Count the number of carbon atoms in the given compound and write the root word for it. Root words upto 10 carbon atoms are tabulated below.
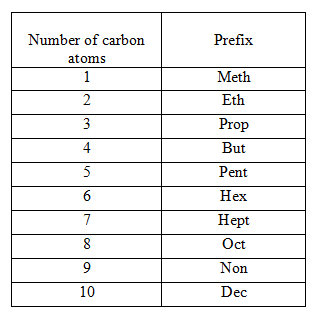
Step II. – If the compound is saturated, add suffix ‘ane’ to the root word, but if it is unsaturated, add suffix ‘ene’ and ‘yne’ for double and triple bonds respectively. e.g. CH₃ CH₂ CH₃ contains three C-atoms, so the root word is ‘prop’ and it contains only single bonds, thus, suffix used is ‘ane’. Hence, the name of this compound is propane.
Similarly, the compound CH₃ CH = CH₂ is named as propene as here suffix ‘ene’ is used for double bond.
Step III. – If functional group is present in the compound, it is indicated in the name of the compound with either a prefix or a suffix .
Chemical Properties Of Carbon Compounds
Some of the important chemical properties of carbon compounds are as follows:
Combustion
All the carbon compounds (including its allotropic forms also) burn in oxygen to give carbon dioxide and water vapours. Heat and light are also released during this process. This reaction i.s called as combustion.
e.g. C + O₂ → CO₂ + Heat +Light
CH₄ + 2O → 2CO₂ + 2H₂O + Heat + Light
2CH₃CH₂OH + 6O₂ → 4CO₂ + 6H₂O + Heat + Light
Further, once carbon and its compounds ignite, they keep o burning without the requirement of additional energy. That’s why, these compounds are used as fuels.
Saturated hydrocarbons give a clean blue flame due to their complete combustion whereas, unsaturated hydrocarbons give a yellow flame with lots of black smoke as they do not undergo complete combustion.
Even saturated hydrocarbons undergoes incomplete combustion in the limited supply of air and gives a sooty flame. The gas stoves used at home has inlets for air so that a sufficiently oxygen rich mixture is burnt to give a clean blue flame.
But sometimes the bottoms of cooking vessels get blackened because the air holes present at the bottom of the vessels gets blocked and fuel [or LPG] do not get enough oxygen.
Therefore, it does not burn properly and undergoes incomplete combustion producing sooty flames which blackened the bottom of the vessels.
Oxidation
It is the reaction involving the addition of oxygen and removal of hydrogen. Alcohols can be oxidised to carboxylic acid by heating them either in presence of oxidising agents like alkaline KMnO₄ (potassium permanganate) or acidified K₂Cr₂O₇ (potassium dichromate).
e.g.
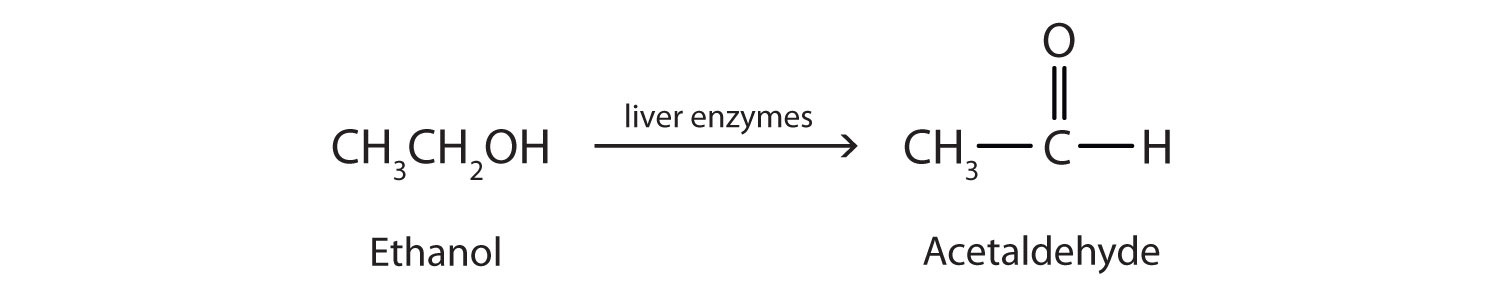
Substances that are capable of adding oxygen to other substances are called oxidising agents.
In general, Alcohol Aldehyde Carboxylic acid Alcohols are converted into carboxylic acid only under complete oxidation. In partial oxidation, alcohols are converted into aldehydes.
Addition Reactions
The reaction in which a reagent completely add to a reactant without the removal of small molecules are called addition reactions.
e.g. addition of hydrogen (hydrogenation) in the presence of catalysts like palladium or nickel, to unsaturated hydrocarbons yield saturated hydrocarbons.

The above reaction is commonly used in the hydrogenation of vegetable oils that are unsaturated compounds] using a nickel catalyst.
Substitution Reactions
The reactions in which a reagent replaces an atom or a group of atoms from the reactant (substrate) are called substitution reactions. These are generally shown by saturated compounds.
Most of the saturated hydrocarbons are fairly insert and unreactive in the presence of most reagents. However, these reactions take place readily in the presence of sunlight e.g.
In the presence of sunlight, chlorine is added to hydrocarbons at a rapid rate. In this reaction, Cl replaces H-atom one by one.
A number of products are usually formed with the higher homologues of alkanes

Fuels And Flames
Fuels
Those carbon compounds which have energy stored in them and burn with heat and light are called fuels. The released energy (heat or light) is utilised for various purposes like for cooking food, running machines in factories etc.
In fuels, the carbon can be in free state as present in coal, coke and charcoal or in combined state as present in petrol, LPG (Liquefied petroleum gas whose main constituent is butane), CNG (Compressed Natural gas, main constituent of which is methane), kerosene, petroleum, natural gas etc.
Those fuels which were formed by the decomposition of the remains of the pre-historic plants and animals (fossils) buried under the earth long ago are called fossil fuels. e.g. coal, petroleum and natural gas.
Coal
It is a complex mixture of compounds of carbon, hydrogen and oxygen and some free carbon along with traces of nitrogen and sulphur. It is formed by the decomposition of plants, ferns and trees which got buried under the earth millions of years ago.
Petroleum
It is a dark viscous foul smelling oil and is also known as rock oil or black gold. It is formed by the decomposition of the remains of extremely small plants and animals buried under the sea millions of years ago.
Flame
A flame is the region where combustion (or burning) of gaseous substances takes place. Depending upon the amount of oxygen available and burning of fuels, flames are of the following two types:
- Blue or Non-luminous Flame : When the oxygen supply is sufficient, the fuels burn completely producing a blue flame and no light is produced. e.g. burning of LPG in gas stove.
- Yellow or Luminous Flame : In the insufficient supply of air, the fuels burn incompletely and produce yellow flame because of the presence of unburnt carbon particles, e.g. burning of wax vapours.
Carbon Compounds – Ethanol
Its common name is ethyl alcohol and formula is C₂H₅OH or CH₃CH₂OH
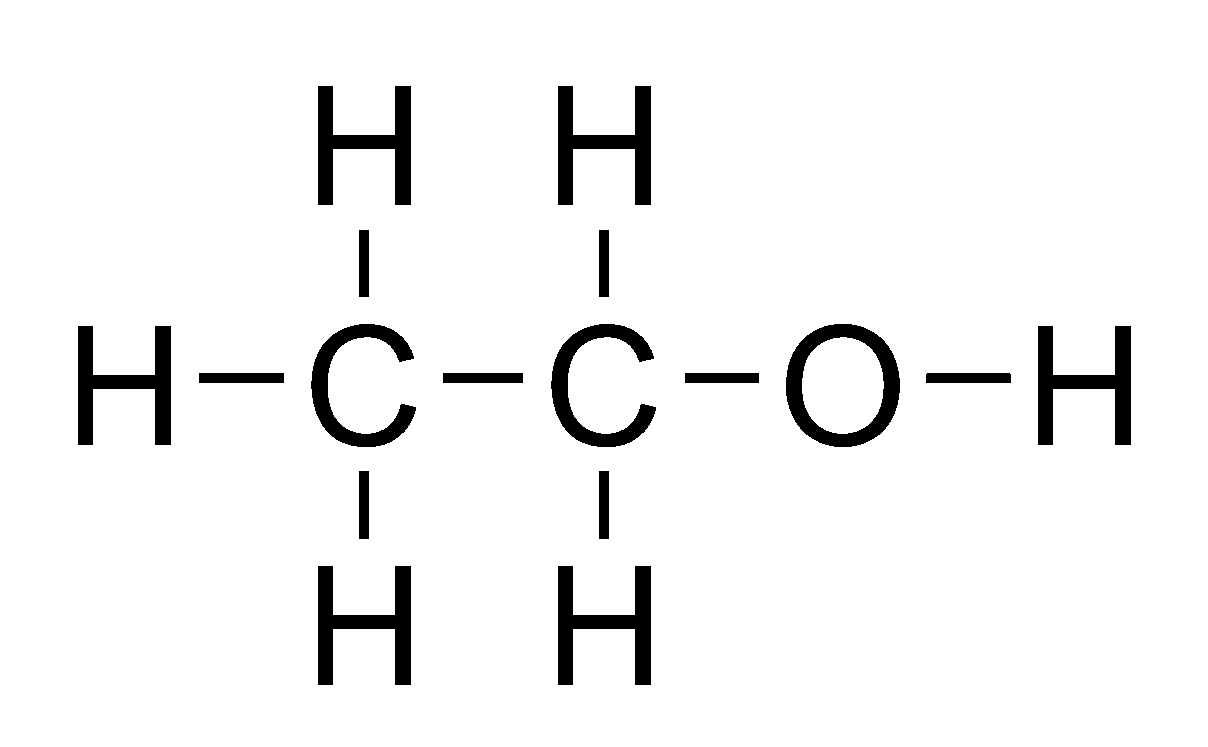
Preparation : Alcohol (ethanol) is obtained by the fermentation of molasses which are obtained from sugarcane juice.
Physical Properties : It is a liquid at room temperature. Its melting point is 156 K and boiling point is 351 K. It is soluble in water in all proportions.
Chemical Properties :
- Reaction with Sodium : Ethyl alcohol reacts with sodium metal leading to the evolution of hydrogen gas alongwith the formation of sodium ethoxide.
- Dehydration : Removal of water molecules from a compound is known as dehydration reaction. When ethanol is heated at 443 K with excess cone. H₂SO₄, the water molecules get removed from it and ethene is obtained.
Thus, in the above reaction, cone. H₂SO₄ act as a dehydrating agent.
Uses : Uses of ethanol are:
- It is used as an active ingredient in all alcoholic drinks.
- It is useful in medicines like tincture of iodine, cough syrups and many other tonics.
- Alcohol is used as an additive in petrol, since it is a cleaner fuel and give rise to only CO₂ and H₂O when burnt in sufficient air.
Carbon Compounds – Ethanoic Acid
It is commonly known as acetic acid. Its formula is CH₃COOH. 5-8% solution of ethanoic acid in water is known as vinegar.
Physical Properties :
Physical properties of ethanoic acid are:
- Its melting point is 290 K.
- During winters it often freezes in cold climates and forms ice like flakes, so it is also called glacial acetic acid. Glacial acetic acid is a trivial name for water free (anhydrous) acetic acid.
- It ‘s a weaker acid than HCI but stronger than alcohol.
Chemical Reactions :
- Acidity : Weak acidity of acetic acid as compared to HCI is because of its low ionisation but is more acidic than alcohol is because of the more stability of ion formed after the removal of a proton (H+). It evolves hydrogen gas when reacts with sodium metal.

- Reaction with a base : It reacts with a base such as sodium hydroxide to give a salt (sodium ethanoate or sodium acetate) and water.

- Esterification : When ethanol (an alcohol) reacts with acetic acid (a carboxylic acid) in the presence of an acid as catalyst, a fruity (sweet) smelling liquid called ester is obtained. This reaction is called esterification.
The ester gets converted back into alcohol and sodium salt of acid when treated with alkali like sodium hydroxide. This reaction is called saponification, as it is used for the preparation of soap.
- Reaction with carbonates and hydrogen carbonates : In the reaction of acetic acid with carbonates or hydrogen carbonates, carbon dioxide gas is obtained. It is an example of acid-base reaction.
Uses Of Acetic Acid
- It is used for making vinegar.
- It is widely used as a preservative in pickles.
- It is used for the synthesis of other compounds like esters.
Soaps And Detergents
Soaps are sodium or potassium salts of long chain carboxylic acids and have general formula![]() where, R=C₁₅H₃₁, C₁₇H₃₅ etc.
where, R=C₁₅H₃₁, C₁₇H₃₅ etc.
Detergents are usually ammonium or sulphonate salts of long chain carboxylic acids. They are also called as soapless soap.
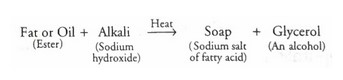
Manufacture Of Soaps And Detergents
Soaps are made from animal fats or vegetable oils by heating it with sodium hydroxide. This process of preparation of soap is called saponification.
Structure of a Soap Molecule
A soap molecule is made up of two parts-a long hydrocarbon part (0r non-ionic part) and a short ionic pan containing -COONa+ group. The long hydrocarbon part is hydrophobic and therefore insoluble in water but soluble in oil.
The ionic portion of the soap molecule is hydrophilic so, soluble in water and insoluble in oil.
Cleansing Action of Soaps (Micelle Formation)
Soap molecules have different properties at their two ends. Its one end is hydrophilic (soluble in water) and other is hydrophobic (soluble in fats or oils).
At the surface of water, the hydrophobic end or tail of soap will be insoluble in water and the soap will align along the surface of water with the ionic end in water and the hydrocarbon ‘tail’ protruding out of water.
Inside water, these molecules show a unique orientation that keeps the hydrocarbon portion out of the water. This is done by forming clusters of molecules in which the hydrophobic tails are in the interior of the cluster and on the surface of duster, ionic ends are present.
This formation of cluster of molecules is called micelle. To wash away the loosened dirt particles in the form of micelles from the surface of the cloth, it is either scrubbed mechanically or beaten or agitated in washing machine.
In the form of a micelle, soap is able to clean, since the oily dirt is being collected in the centre of micelle. Micelles stay as colloids in the solution and does not come together to precipitate due to ion-ion repulsion. Hence, the dirt suspended in the micelles is also easily rinsed away.
NCERT Notes for Class 10 Science Chapter 4 Carbon and its Compounds
Question 1. What would be the electron dot structure of carbon dioxide which has the formula CO2?
Answer: Carbon has an atomic number of 6 (Z), and its electronic configuration is 2, 4. There are four valence electrons in carbon.
There are six valence electrons on every oxygen atom (Z = 8). (2, 6). To finish its octet, the carbon atom shares its four valence electrons with the two oxygen atoms’ four electrons :
So, in the carbon dioxide molecule, the carbon atom is connected to two oxygen atoms on both sides by two pairs of shared electrons, making double bonds on both sides. By sharing electrons, both carbon and oxygen can complete their octet.
Question 2.What would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur?
Answer: The atomic number (Z) of sulphur is 16, and its electronic configuration is 2, 8, 6. The sulphur atom has six valence electrons. The chemical formula of sulphur molecule is S8.
Each atom of sulphur is connected to two other similar atoms on both sides by a single covalent bond. This makes up its octet. The molecule looks like a ring, which is also called a crown shape.
Ring structure of S8 molecule Crown shape of S8 molecule
Question 3. How many structural isomers you can you draw for pentane?
Answer: Pentane is made up of five carbon atoms (C5H12). It can be a straight chain or two chains that branch off from each other. The hydrocarbon, which is an alkane, can have three different shapes.
Question 2. What would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur?
Answer: Sulphur has an atomic number (Z) of 16, and its electronic configuration is 2, 8, 6. There are six valence electrons on the sulphur atom. The chemical formula of sulphur molecule is S8.
Each atom of sulphur is linked to two other atoms of the same kind on either side by a single covalent bond. This makes up its octet. The molecule is in the shape of a ring, which is also called a “crown.”
Ring structure of S8 molecule Crown shape of S8 molecule
Question 3. How many structural isomers you can you draw for pentane?
Answer: Five carbon atoms make up the structure of pentane (C5H12). It can be both a straight chain and a chain with two branches. The alkane hydrocarbon has three different shapes, or isomers.
Question 4. What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
Answer:
- Catenation: Catenation is the unique ability of carbon to link itself together. Covalent bonds can connect any number of carbon atoms to each other. This is because C—C bonds are stable because the carbon atom is so small.
- Linking of carbon with other atoms : Carbon is a tetravalent element, which means it can easily share electrons with other atoms like hydrogen, oxygen, nitrogen, sulphur, etc.
Question 5. What will be the formula and electron dot structure of cyclopentane? (CBSE 2013)
Answer: Cyclopentane is a cyclic compound with formula C5H12 The structure of the compound may be represented as :
Question 6. Draw the structures of the following compounds :
(i) Ethanoic acid
(ii) Bromopentane
(iii) Butanone Are structural isomers possible for bromopentane ?
Answer:
Bromopentane has a chain of five carbon atoms. It can exist in a number of forms which are structural isomers.
The structural isomers (i), (ii) and (iii) which differ in the position of the Br atom are known as position isomers.
Chain isomers are the structural isomers (iv), (v), and (vi) that differ in how the carbon atoms are arranged in the chain.
In the IUPAC name, the name of the prefix Bromo comes before the name of the prefix methyl. In fact, the different prefixes are named in alphabetical order.
Question 7. How would you name the following compounds ?
Answer:
(i) Bromoethane
(ii) Hex-l-yne
(iii) Methanal
Question 8. Why is the conversion of ethanol into ethanoic acid an oxidation reaction ?
Answer: Ethanoic acid (CH3COOH) has one oxygen atom more and two hydrogen atoms less than ethanol (C2H5OH).
In general,
- Loss of hydrogen is known as oxidation.
- Gain of oxygen is known as oxidation.
Therefore, it is an oxidation reaction.
Question 9. A mixture of ethyne and oxygen is used for welding. Can you tell why a mixture of ethyne and air is not used ?
Answer: When ethyne is burned with oxygen, a lot of light and heat are made. The heat that is made can be used for gas welding, which is usually done to put together small pieces of broken iron items.
Most of the air is made up of a mix of nitrogen (4 parts) and oxygen (1 part). We know that nitrogen gas doesn’t help fires start. This means that oxygen is the only thing that will help ethyne burn in air. Because of this, it is always best to burn ethyne with oxygen.
Question 10. How would you distinguish experimentally between an alcohol and a carboxylic acid ?
Answer:
The distinction can be made by the following tests:
- Dip a strip of blue litmus separately in two glass tubes of alcohol and carboxylic acid. In the tube with carboxylic acid, the colour will change to red. In the tube with alcohol, the colour will not change.
- Add a small amount of solid sodium hydrogen carbonate (NaHCO3) in both the tubes. In the tube with carboxylic acid, there will be a strong fizzing sound and bubbles, but not in the tube with alcohol.
Question 11. What are oxidising agents ?
Answer: Oxidizing agents are substances that release oxygen on their own or when they react with another substance. This is how oxidation reactions happen. Ozone, bromine water, a mixture of potassium dichromate and sulphuric acid, or a mixture of potassium permanganate and sulphuric acid are all common oxidising agents.
Question 12. Would you be able to check if water is hard by using a detergent ?
Answer: No, it can’t happen. In fact, detergents make foam in any kind of water, hard or soft.
So, you can’t tell the difference between the two. But soaps can be used to do this job.
Question 13. People use a variety of methods to wash clothes. Usually after adding soap, they beat the clothes on a stone or beat them with a paddle, scrub with a brush or the mixture is agitated in a washing machine Why is this agitation necessary to get clean clothes ?
Answer: Soap or detergent is used to wash because it makes it easier for the oil drops carrying dirt particles and the water to mix together. All of the methods that have been suggested loosen the bonds between the dust or oil particles and the fabrics of clothes. The agitation helps the clothes get clean.
Question 1. Ethane, with the molecular formula C2H6 has :
(a) G covalent bonds
(b) 7 covalent bonds
(c) 8 covalent bonds
(d) 9 covalent bonds
Answer:
(b). The molecule has seven covalent bonds.
Question 2. Butanone is a four-carbon compound with the functional group
(a) carboxylic acid
(b) aldehyde
(c) ketone
(d) alcohol
Answer:
(c). The functional group is ketone (>C=0) also known as one.
Question 3. While cooking, if the bottom of the vessel is getting blackened on the outside, it means that
(a) the food is not cooked completely
(b) the fuel is not burning completely
(c) the fuel is wet
(d) the fuel is burning completely
Answer:
(b). The fuel isn’t completely burning. The outside of the vessel becomes black because smoke contains unburned particles.
Question 4. Explain the nature of the covalent bond using the bond formation in CH3Cl.
Answer: The molecule of chloromethane (CH3Cl) consists of three elements i.e., carbon (Z = 6) hydrogen (Z = 1) and chlorine (Z = 17). Carbon has four valence electrons (2, 4); hydrogen has one (1); and chlorine has seven electrons in the valence shell (2, 8, 7).
For its octet to be complete, carbon shares three of its valence electrons with three hydrogen atoms and one with the electron of a chlorine atom. A covalent molecule’s structure can be written as follows:
Question 5. Draw the electron dot structures for
(i) ethanoic acid
(ii) H2S
(iii) propanone
(iv) F2
Answer:
Question 6.
What is homologous series ? Explain with an example.
Answer: A group of compounds with the same chemical properties and the same functional group. Any two consecutive members of the same group differ in their molecular formula by a — CH2 group and their molecular mass by 14 u.
For example, the boiling points of alkanes go in the following order:
Question 7. How can ethanol and ethanoic acid be differentiated on the basis of their physical and chemical properties? (CBSE Delhi 2011)
Answer:
Distinction based on physical properties:
- Smell: Ethanol has a pleasant smell that people call “the alcoholic smell.” Ethanoic acid smells like vinegar.
- Boiling points : Ethanol’s boiling point (351 K) is lower than that of ethanoic acid (391 K).
- Litmus test: Ethanol is neutral, so it doesn’t change the colour of a litmus strip, whether it’s blue or red. Ethanoic acid is acidic, and when a blue litmus strip is dipped in it, it turns red.
Distinction based on chemical properties:
- Action with sodium hydrogen carbonate: When a small amount of sodium hydrogen carbonate is added to ethanoic acid, carbon dioxide gas bubbles up quickly. But this doesn’t happen when ethanol is used. CH3COOH + NaHCO3 ———> CH3COONa + CO2 + H2O
- Action with caustic alkalies: Both sodium hydroxide (NaOH) and potassium hydroxide (KOH) react with ethanoic to make a salt and water. Neither of these things reacts with ethanol..
Question 8. Why does micelle formation take place when soap is added to water ? Will a micelle be formed in other solvents such as ethanol also ? (CBSE Delhi 2011)
Answer:
Soap has the formula RCOO–Na+ , where R is an alkyl group, which is a long chain of carbon atoms with fifteen or more. Now, dirt particles in oil drops don’t mix with water. Soap helps them mix by lowering the friction between the two surfaces.
In fact, it acts like a bridge between oil drops and water, with the hydrophobic end of the alkyl part pointing toward the oil drop and the hydrophilic end of the COONa part pointing toward the water. This process is called micelle formation.
Soap makes it easier for oil and water to mix together in a stable way. Since soap dissolves in ethanol and other organic solvents that are similar to it, they do not help form micelles.
Question 9. Why are carbon and its compounds used as fuels in most cases?
Answer: When carbon burns in oxygen or air, carbon dioxide gas is made. The reaction makes a lot of heat. That is why different kinds of coal are used as fuels. Hydrocarbons are the most important carbon compounds. Just like carbon, hydrogen burns easily in air or oxygen to make water and heat.
The hydrocarbon methane (CH4) is a constituent of natural gas. Propane (C3H8) and butane (C₄H₁₀) are present in liquid petroleum gas (L.P.G.). Both gasoline and kerosene have different kinds of hydrocarbons. So, these are used to make fuels.
Question 10. Explain the formation of scum when hard water is treated with soap. (CBSE Delhi 2011)
Answer: Soap is basically sodium or potassium salts of higher fatty acids. Hard water has the salts of the Ca2+ and Mg2+ ions in it. When soap is mixed with hard water, the calcium and magnesium salts that go with it are made. These are in the form of “precipitates,” which is another word for “scum.”
Question 11. What change will you observe by testing soap with litmus paper (blue or red)?
Answer: When soap is dissolved in water, alkali NaOH or KOH is made. This makes the solution alkaline. The solution changes the colour of the red litmus to blue. However, the solution does not change the colour of blue litmus.
Question 12. What is hydrogenation? What is its industrial application ?
Answer: Catalytic hydrogenation is the process of adding hydrogen to an unsaturated hydrocarbon in the presence of a metal catalyst.
Hydrogenating vegetable oils, also called edible oils, like ground nut oil, cotton seed oil, etc., is a great way to use the reaction. These are also called cooking oils, and their molecules are unsaturated because they have at least one C=C bond.
When hydrogen gas is passed through oil with a nickel catalyst present, the double bond changes into a single bond. Because of this, the unsaturated oil changes into saturated, solid fat. Vegetable ghees like Dalda are saturated and are made by hydrogenation with a catalyst.
Question 13. Which of the listed hydrocarbons undergo addition reactions: C2H6, C3H8, C3H6, C2H2 and CH4?
Answer: In order that a hydrocarbon may undergo addition reaction, it must be unsaturated in nature. It must be either an alkene (C=C) with general formula CnH2n or an alkyne (C = C) with general formula CnH2n-2. Out of the list of the hydrocarbons given:
- C3H6 (Propene) is an alkene with C=C bond. It corresponds to general formula CnH2n (n = 3)
- C2H2 (Ethyne) is an alkyne with C = C bond. It corresponds to general formula CnH2n-2 (n = 2).
Both these hydrocarbons take part in addition reactions. For example, they react with hydrogen upon heating to 473 K in the presence of Nickel catalyst to form corresponding alkanes.
Question 14. Give a test that can be used to differentiate between butter and cooking oil.
Answer: Butter is naturally saturated, but cooking oil is not. This means that at least one C=C bond is present in the compounds that make up cooking oil, but there is no such bond in butter.
By reacting with bromine water or bromine dissolved in carbon tetrachloride, you can tell them apart. The yellow colour of bromine comes out of cooking oil, but not from butter.
Question 15. Explain the mechanism of cleansing action of soap.
Answer:
Cleansing action of Soaps and Detergents
Soaps and detergents both clean things in the same way. There are two main parts to them. These are nonpolar hydrocarbon chains that don’t like water, or are hydrophobic (the word “phobia” means “repulsion” or “hatred”), and their salt is a polar carboxyl group that likes water, or is hydrophilic (philic stands for love or attraction). The first part is called the molecule’s “tail,” and the second part is called its “head.”
In order to understand the cleansing action of both soaps and Let’s try to figure out why clothes get dirty before we use detergents. The first thing that makes them oily is the sweat that comes out of the skin and the organic matter that is in the air. When drops of oil land on dust, the clothes get dirty.
In order to wash these, they are dipped in water and soap or detergent is applied. In solution, it dissociates to give carboxylate ions (RCOO ) or sulphonate ions (RSO3–) and the cations (Na+).
In the carboxylate ion, the alkyl portion which contains a long chain of hydrocarbons is a tail pointing towards the oil drops while the COO– portion is the head directed towards water.
In a detergent it is the SO3– portion which points towards water. This is clear from the figure, where the solid circles (•) represent the polar groups and the wavy lines () represent the alkyl parts. Soap and detergent molecules line up in a certain way in water.
In reality, they form a group of molecules with the hydrophobic or alkyl part in the middle and the ionic or polar part on the outside, as shown in the figure.
This is called a micellear formation or just a micelle. Soaps and detergents help oil and water mix together into a stable emulsion by acting as a link between the two.
The oil droplets and dirt particles separate from the fibres of the clothes and go into the emulsion. This gets rid of any dust or dirt on the clothes.