CBSE Class 10 Science Notes Chapter 1 Chemical Reactions and Equations
Welcome to our blog post on CBSE Class 10 Science! In this post, we have compiled a comprehensive collection of notes, question bank, and videos to help you master the chapter.
Our notes are written in an easy-to-understand format and are based on the latest CBSE curriculum. The question bank includes a wide range of questions, covering all the important topics in the chapter. And our videos are designed to make the learning process interactive and engaging.
We hope that this post will be a valuable resource for you as you prepare for your exams. So, let’s get started and dive into the world of history!
Chemical Reactions
Chemical Reactions
A chemical reaction is a transformation in which one or more substances or reactants react to form new substances with completely different properties.
The reactants (substances that undergo chemical change during the reaction) are referred to as reactants, and the new species formed as a result of the reaction are referred to as products (the new substances formed during the reaction).
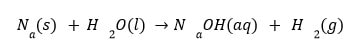 The products of the above chemical reaction are sodium hydroxide and hydrogen, while the reactants are sodium and water.
The products of the above chemical reaction are sodium hydroxide and hydrogen, while the reactants are sodium and water.
NCERT Notes for Class 10 Science Chapter 1 Chemical Reactions and Equations
Science is a subject that explains how the world around us is made of Chemical reactions that are used to explain the various processes that happen around us. From rust to decomposition, chemical reactions provide a more in-depth insight into how molecular interaction and changes occur. In other words, chapter 1 of CBSE class 10 Science explains how a substance changes form.
CBSE Class 10 Science notes will assist students in studying the topic thoroughly and clearly.
These CBSE Class 10 Science notes were written by subject experts who made the study material very basic, both in terms of language and format.
Identification Of Chemical Reaction
Identification Of Chemical Reaction
The Following Observations Can Be Used To Identify A Chemical Reaction:
- State change
- Color change
- Gas evolution
- Temperature change
- Precipitation formation)
Writing A Chemical Equation
Writing A Chemical Equation
An arrow (—>) placed between reactants and products in a chemical equation depicts the change from reactants to products. Reactants are written on the left-hand side (LHS) of the arrow with a plus sign (+) between them. Similarly, products are written on the right-hand side (RHS) with a plus sign (+) between them.
![]()
The arrowhead points to the products and indicates the reaction’s direction; for example, the reaction between magnesium (Mg) and oxygen (O2) that produces magnesium oxide can be written as:
The number of magnesium and oxygen atoms on both sides of the equation are not equal in the above equation. The skeletal chemical equation is a type of unbalanced equation.
Balanced Chemical Equations
Balanced Chemical Equations
On both sides of the equation, a balanced chemical equation has the same total number of atoms for each element. The law of conservation of mass is used to balance a chemical equation.
The law of conservation of mass states that “mass cannot be created or destroyed during a chemical reaction.” Similarly, before and after a chemical reaction, the number of atoms in each element remains constant.
The hit-and-trial method for balancing chemical equations is named after the fact that we try to balance the equation using the smallest whole number coefficient. The number of atoms in each element remains constant before and after a chemical reaction in this method.
Balancing Of A Chemical Equation
Balancing Of A Chemical Equation
A chemical equation must be balanced in several steps.
These are the steps to take:
Step (a) Write An Unbalanced Equation With The Formulae Enclosed In Brackets.
![]()
Step (b) In an imbalanced equation, make a list of the number of atoms of different elements that are present.
Element | Number of Atoms in Reactants (LHS) | Number of Atoms in Products (RHS) |
Na | 1 | 1 |
H | 2 | 3 |
O | 1 | 1 |
C) Balancing The First Element : It is clear from the table above that only the hydrogen atoms are out of balance. As a result, we begin by trying to balance it.
Atoms of H | In Reactants | In Products |
Na | 1 | 1 |
H | 2 | 3 |
O | 1 | 1 |
The equation now,
![]()
Step (D) Equilibration Of The Second Element: We examine the obtained equation and select another unbalanced element. Na remains unbalanced in the previous equation.
To maintain a balance in the number of Na-atoms.
Atoms of Na | In Reactants | In Products |
Initially | 1 (in na) | 2 (in NaOH) |
To balance | 2 x 1 | 2 |
Step (E) Balancing Other Elements: Further examination of the reaction reveals that no element is out of balance. This is referred to as the hit-and-trial method of balancing chemical equations.
Step (F) Verifying The Equation’s Correctness: To guarantee that the equation is correct, we tabulate the number of atoms in each element separately.
Element | Number of Atoms in Reactants (LHS) | Number of Atoms in Products (RHS) |
Na | 2 | 2 |
H | 4 | 4 |
O | 2 | 2 |
Making A Chemical Equation More Informative
Making A Chemical Equation More Informative
The following facts about chemistry remain unexplained. Step (d) demonstrates the equation:
- Substances’ physical states
- Reaction conditions
- Energy evolution/absorption
Some of these limitations of a chemical equation can be overcome by including the following symbols or information:
- The physical states of reactants and products are denoted by the symbols (s) for solid, (1) for liquid, (g) for gas, and (aq) for aqueous solution, respectively. If the reactant or product is dissolved in water, the term aqueous (aq) is used.
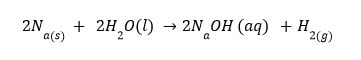 Instead of the symbol, an arrow pointing downward (↓) can be used to represent precipitation (s).
Instead of the symbol, an arrow pointing downward (↓) can be used to represent precipitation (s).
Similarly, rather than using the symbol, the gaseous state of an evolved gas can be represented (g)
by an arrow pointing upward(🠑) I
The chemical equation for magnesium reacting with dilute sulphuric acid is
- The reaction’s specific conditions, such as temperature, pressure, and catalyst, are specified above or below the arrow in the chemical equation.

- Heat evolution or absorption can be indicated by writing [+Heat] on the right-hand side or the left-hand side of the equation, respectively.
Types Of Chemical Reactions - Combination Reaction
Types Of Chemical Reactions
Chemical reactions are classified according to the type of chemical changes that occur. These are the reactions:
- Combination Reaction
A combination reaction exists when 2 or more reactants react vigorously to form a single product.
- Calcium Oxide (Quick Lime) Reacts Vigorously With Water To Form Calcium Hydroxide (Slaked Lime).
The reaction is highly exothermic, as it generates a great deal of heat.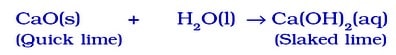
- Burning Of Coal.
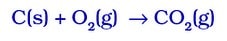
- Reaction Between Hydrogen Gas And Oxygen Gas To Form Water.

Decomposition Reaction
- Decomposition Reaction
A decomposition reaction occurs when a single reactant degrades into two or more products.
A decomposition reaction occurs when a single reactant breaks down into two or more products. This is the opposite reaction of the combination reaction.
These reactions are classified into three types according to the type of energy required for the reaction:
- Thermal Decomposition: These reactions utilise energy in the form of heat to cause the reactant to decompose.
- Calcium carbonate decomposes upon heating to form calcium oxide and carbon dioxide. Calcium oxide is used to make cement.

- Ferrous sulphate, the green crystals FeSO₄ .7H₂O, crystallises and dehydrates to form FeSO₄, which decomposes to form a ferric oxide, sulphur dioxide,SO₂, and sulphur trioxide, SO₃. While ferric oxide is a solid, sulphur dioxide and sulphur trioxide are gases.
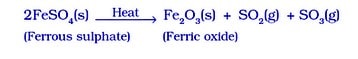
- Lead nitrate decays upon heating to form yellow lead monoxide, nitrogen dioxide, and oxygen gas.

- Calcium carbonate decomposes upon heating to form calcium oxide and carbon dioxide. Calcium oxide is used to make cement.
- Electrolysis: Electrical energy is used to break down the reactant molecules in these reactions.
- Water decomposes into oxygen and hydrogen when an electric current is passed through it.

- When molten sodium chloride is exposed to an electric current, it decomposes into sodium metal and chlorine gas.

- Water decomposes into oxygen and hydrogen when an electric current is passed through it.
Photolysis Is A Term That Refers To The Photochemical Decomposition Of Matter
Photolysis Is A Term That Refers To The Photochemical Decomposition Of Matter.

These reactions rely on light energy for decomposition. (a) When silver chloride is exposed to sunlight, it decomposes to form silver metal and chlorine gas.
Displacement Reaction
Displacement Reaction
This reaction is classified into two types:
Single Displacement Reaction
Single displacement is a type of chemical reaction in which an element reacts with a compound and takes the place of another element in that compound.
- Because zinc is more reactive than copper, it displaces copper from CUSO₄ solution, forming zinc sulphate and copper metal.
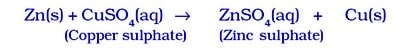
- Similarly, because iron is more reactive than copper, it displaces copper from a solution of copper sulphate in water.
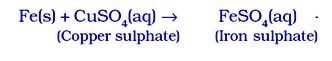
Double Displacement Reaction :
The double displacement reaction occurs when two distinct ions or groups of atoms in the reactant molecules are displaced by one another. Additionally, it is referred to as a precipitation reaction because precipitate is produced in such reactions.
(a) When sodium sulphate is added to barium chloride, a curdy white precipitate of barium sulphate and sodium chloride solutions form.

(b) When sodium bromide is added to silver nitrate solution, a yellow precipitate of silver bromide and sodium nitrate solutions are formed.
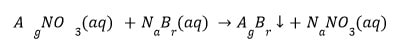
Oxidation And Reduction Reactions
Oxidation And Reduction Reactions
Oxidation
It is defined as the addition of oxygen to a substance.
Or
The process of removing hydrogen from a substance.
Or
The process by which a substance’s electrons are lost (s).
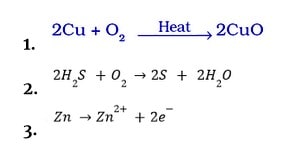
Reduction
It is defined as the process of removing oxygen from a substance.
Or
The addition of hydrogen to a substance.
Or
The method by which a substance acquires electrons (s).
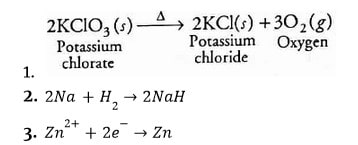
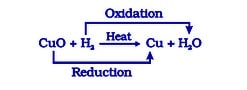
Redox Chemical Reactions
Redox Chemical Reactions
Redox reactions are those in which oxidation and reduction occur concurrently.
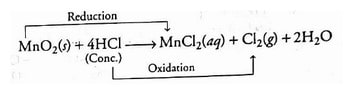
- The copper (II) oxide is reduced in the following reaction. In contrast, oxygen is added to hydrogen and oxidised.
- Within. Following the reaction, HCl is reduced to Cl₂, while MnO₂ is oxidised to MnCl₂.
Exothermic And Endothermic Reactions
- Exothermic And Endothermic Reactions
A reaction can be exothermic or endothermic depending on whether heat is evolved or absorbed during the course of the reaction.
- Exothermic Reactions
Exothermic reactions (combustion reactions) are those that involve the evolution of heat.
The formation of products is called exothermic reactions. Exothermic processes such as respiration occur during this process.
Composting vegetable matter is also an exothermic reaction.
![]()
- Burning Of Natural Gas
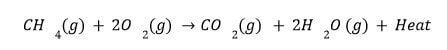
- Burning Of Magnesium Ribbon
![]()
Thermodynamic (Endothermic) Reactions:
Thermodynamic (Endothermic) Reactions:
Endothermic reactions are those that occur as a result of the absorption of heat/energy (either in the form of light or electricity).
The process of photosynthesis is endothermic.
All decomposition reactions are endothermic, requiring energy in the form of heat, light, or electricity to decompose the reactants.
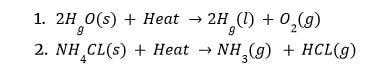
Effect Of Oxidation Reactions In Everyday Life
Effect Of Oxidation Reactions In Everyday Life
Corrosion
Corrosion is the process by which metals are gradually eroded by the reaction of air, water, and chemicals in the atmosphere.
When iron articles are new, they are shiny, but after a period of time, they become coated with a reddish-brown powder. This is commonly referred to as iron rusting.
Other examples of corrosion include the black coating on silver and the green coating on copper.
Corrosion Effects:
Corrosion Has The Following Effects:
Corrosion wreaks havoc on automobile bodies, bridges, iron railings, and ships, as well as on all metal objects, particularly those made of iron.
Corrosion is, in most cases, a wasteful process. Each year, the country wastes tonnes of various metals, most notably iron. As a result, corrosion prevention is critical.
Rancidity
It is the slow oxidation of oil and fat (which are inherently volatile) found in food materials, resulting in a change in their smell and taste.
Preventing Rancidity Involves The Following Steps:
Preventing Rancidity Involves The Following Steps:
- Keeping food materials in airtight containers:
- Low-temperature refrigeration of cooked food.
- Packing food items such as potato wafers and other similar items in packets containing nitrogen gas rather than air. 7
- Prevent cooking food and food materials from being exposed to direct sunlight.
- By supplementing with BHA (Butylated Hydroxy Anisole) and BHT (Butylated HydroxyToluene) antioxidants.
NCERT questions & answers from Chemical Reactions and Equations
Why should magnesium ribbon be cleaned before burning in air?
Most magnesium ribbon has a coating of basic magnesium carbonate on its surface. It is a mixture of magnesium hydroxide and magnesium carbonate, and it slowly builds up on the surface of the metal when moist air moves over it.
The coating or layer keeps the metal from catching fire when flame comes in contact with it. Before burning the ribbon in air to remove the magnesium oxide layer, the surface should be cleaned well, preferably with sandpaper.
Write the balanced equations for the following chemical reactions : (i) Hydrogen + Chlorine ———> Hydrogen chloride (ii) Sodium + Water ———> Sodium hydroxide + Hydrogen (iii) Barium chloride + Aluminium sulphate ———> Barium sulphate + Aluminium chloride.
Answer: The balanced equations are written for the symbol equations and not for word equations.
(i) H2+Cl22HCL
(ii) 2NA+2H2O2NaOH+H2
(iii) 3BaCl2+Al2(SO4)33BaSO4+2AlCl3
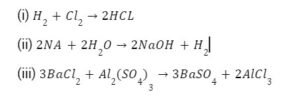
Write the balanced equations with state symbols for the following reactions : (i) Solutions of barium chloride and sodium sulphate in water react to give insoluble barium sulphate and solution of sodium chloride. (ii) Sodium hydroxide solution (in water) reacts with hydrochloric acid solution (in water) to produce sodium chloride (in water) and water.
Answer:
The symbol equations in balanced form for the reactions are :
(i) BaCl2(aq)+Na2SO4(aq)BaSO4(s)+2NaCl(aq)
(ii) NaOH(aq)+HCL(aq)NacL(aq)+H2O(l)
What is a balanced chemical equation ? Why should chemical equations be balanced ? (CBSE 2013)
Balanced chemical equation. A chemical equation is said to be balanced if :
- On both sides of the equation, the atoms of the different elements are the same.
- The equation is molecular, which means that if gases are part of it, they must be in molecular form. (eg., H2, O2, N2, Cl2 etc.)
It is important to keep chemical equations in balance. To follow the law of conservation of mass, the chemical equations must be balanced. According to the law, the mass of the reactants and the mass of the products must be the same in a chemical reaction. This is only possible if the number of atoms of the different elements in the reactants and the products are the same.
Explain the following terms with one example of each. (a) Corrosion (b) Rusting.
Corrosion is the chemical process by which the surface of some metals slowly wears away when they are left out in the open for a long time.
When we open the hood of a car after a long time, we often find a buildup around the battery’s terminals. This is because the terminals have rusted. Corrosion shows up as a black layer on the surface of silver and a green layer on the surface of copper.
Corrosion in iron is called “rusting.” Rust is a chemical that is soft and has small holes in it. It is brown and is made when chemicals react with moist air.
(containing CO2 and H2O) on iron. It is basically an oxidation reaction and formula of rust is Fe2O3.xH2O. It is very slow in nature and once started keeps on.
Corrosion and rusting are both very dangerous and cause damage to buildings, railroad tracks, cars, and other things made of metal. We hear a lot about old buildings that have fallen down on their own, killing people and destroying property. This is because the iron used to build the buildings, especially the roof, is rusting.
Corrosion also does constant damage to marble statues, which are made of calcium carbonate.
(CaCO3). Both sulphuric acid and nitric acid present in the rain water dissolve calcium carbonate to form calcium sulphate and calcium nitrate respectively.
CaCO3 + H2SO4 ———— > CaSO4 + CO2 + H2O
CaCO3 + 2HNO3 ———— > Ca(NO3)2 + CO2 + H2O
In addition to this, traces of hydrogen sulphide gas (H2S) present in atmosphere form black stains on these statues due to calcium sulphide which is black in colour.
CaCO3 + H2S ———–> CaS + H2O + CO2
Corrosion has caused huge damage to our historical monuments including ‘Taj Mahal’ which is regarded as the eighth wonder. Marble is chemically CaCO3. Polluted air contains both H2SO4 and HNO3 along with traces of H2S gas. They react chemically with CaCO3 as shown above.
So, this historical building, which is India’s pride, is always getting worse. We must do everything we can to protect the Taj Mahal and other treasures like it. Corrosion, also called rusting, only happens to metals. In chapter 3 on Metals and Non-Metals, we’ll learn more about corrosion and how to stop it.