Study Notes of Acids Bases and Salts
Acids
Acids are those chemical substances which have a sour taste and change the colour of blue litmus to red. Some common fruits such as unripe mango, lemon, orange, tamarind etc., are sour in taste.
This suggests that these fruits contain acids. Some commonly used acids are hydrochloric acid (HO), sulphuric acid (![]() ), nitric acid (
), nitric acid (![]() ) etc.
) etc.
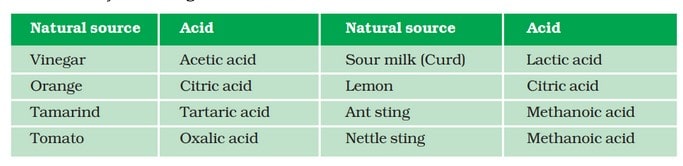
Some naturally occurring acids are:
Chemical Properties of Acids
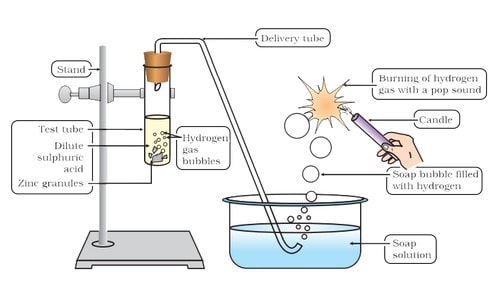
(i) Reaction with metals Acids like dilute HCl and dilute ![]() , react with certain active metals like zinc (Zn), iron (Fe) etc., to form salt and evolve
, react with certain active metals like zinc (Zn), iron (Fe) etc., to form salt and evolve ![]() gas.
gas.
Thus, these acids or substances containing these kinds of acids should not be kept in metal containers.
Metal + Dilute acid –> Salt + Hydrogen gas
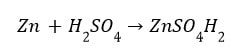
(ii) Reaction with metal carbonate and hydrogen carbonate Limestone, chalk and marble are different forms of calcium carbonate.
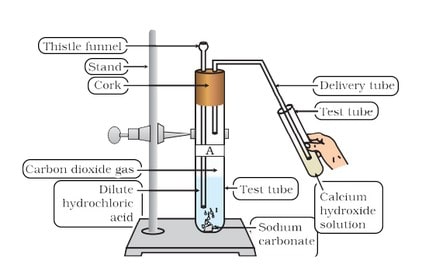
Acids react with metal carbonates and hydrogen carbonates (bicarbonate) to produce their corresponding salt, carbon dioxide gas and water.
Metal carbonate / Metal hydrogen carbonate + Acid —> Salt + Carbon dioxide + Water
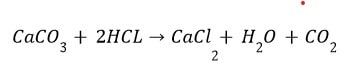
The carbon dioxide gas is released with a brisk effervescence.
Test for ![]() gas When
gas When ![]() gas is passed through lime water, it turns milky due to the formation of white precipitate of
gas is passed through lime water, it turns milky due to the formation of white precipitate of ![]()
But if ![]() is passed in excess, milkiness disappears due to the formation of Ca(HCO3 ) 2 which is soluble in water.
is passed in excess, milkiness disappears due to the formation of Ca(HCO3 ) 2 which is soluble in water.
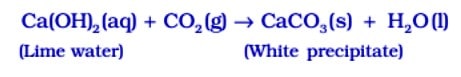
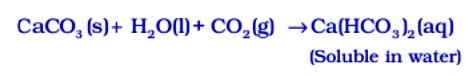
(iii) Reaction with metal oxides Acids react with certain metal oxides (being basic in nature also called metal oxides) to form salt and water.
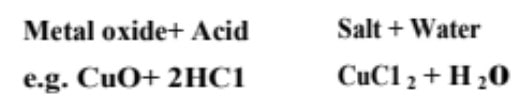
Bases
Bases are those chemical substances which are bitter in taste, soapy to touch and turn red litmus to blue. sodium hydroxide (NaOH), calcium hydroxide Ca(OH) 2 etc.
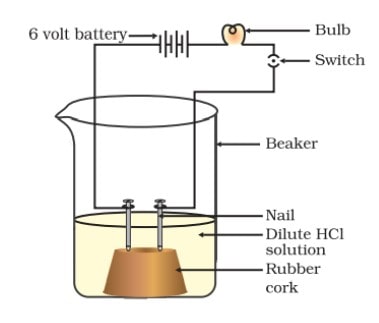
Chemical Properties of Bases
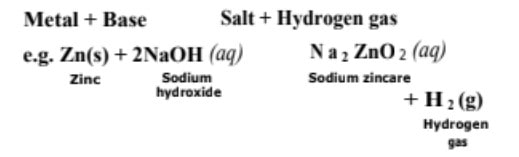
(i) Reaction with metals Strong bases react with active metals should not be kept in metal containers (active metals). metals to produce hydrogen gas. Thus, these bases
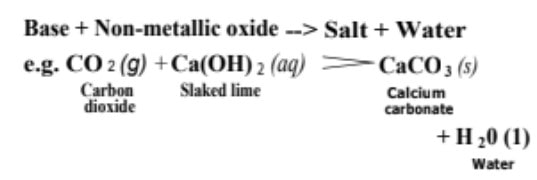
(ii) Reactions with non-metallic oxide Bases react with non-metallic oxides (being acidic in nature also called acidic oxides) to produce salt and water.
This reaction proves that non-metallic oxides are acidic in nature.
Acids/ Bases in Water Solution
In the presence of water, acids give H⁺ ions . As H⁺ ions cannot exist alone so it combines with water molecules and forms H₃0 + (Hydronium ion).
So, we can say in the presence of water acids give H⁺ ions or H₃0 + ions. In the same way, in presence of water, bases give OH ions.
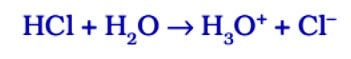
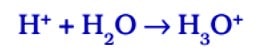

Reaction Between Acids and Bases
Acids react with bases to produce salt and water. In this reaction, an acid neutralises a base, i.e. acid nullifies or reduces the effect of a base or vice-versa, thus the reaction is known as neutralisation reaction.
In general, neutralisation reaction can be written as :
 Here, H represents hydrogen atom and M represents metal atom.
Here, H represents hydrogen atom and M represents metal atom.
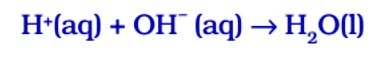

Effect of Dilution on an Acid or Base
Mixing of an acid or base with water is called dilution. It results in a decrease in the concentration of ions (H₃0 + /OH⁻ ) per unit volume and the acid or base is said to be diluted.
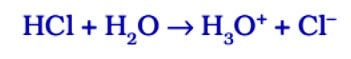
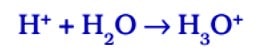

Dissolving an acid or a base in water is a highly exothermic (heat generating) reaction, so care must be taken while doing it. The acids must always be added slowly to water with constant
Stirring. Water should not be added to concentrated acid because if water is added, the heat generated may cause the mixture to splash out and cause burns.
Indicators
Indicators are the substances that change their colour or odour when added into an acid or an alkaline solution. Indicators can be classified in the following ways :
Natural Indicators
These indicators are found in nature in the plants, litmus solution is a purple colour dye extracted from the lichen plant belonging to the division “Thallophyta”.
Some Natural Indicators with Characteristic Colours
| Indicator | Colour in Acidic Medium | Colour in Alkaline Medium |
| Litmus | Red | Blue |
| Red cabbage juice (from leaves) | Red | Green |
| Turmeric juice (haldi) | Yellow | Reddish brown |
| Flowers of Hydrangea plant | blue | Pink |
Synthetic Indicators
The indicators which are synthesised in the laboratory or industry are known as synthetic indicators, methyl orange, phenolphthalein, methylene blue and methyl red are synthetic indicators.
Some Synthetic Indicators with Characteristic Colours
| Indicator | Colour in Acidic Solution | Colours in Basic Solution | Colour in Neutral Solution |
| Phenolphthalein | Colourless | Pink | Colourless |
| Methyl orange | Red | Yellow | Orange |
Olfactory Indicators
Those substances whose odour changes in acidic or basic medium are called olfactory indicators. Vanilla extract and onion can be used as olfactory indicators.
The smell of these two indicators can be detected in the presence of acid only but not in the presence of base.
Universal Indicators
To judge how strong a given acid or base is, a universal indicator is used, which is a mixture of several indicators. It shows different colours at different concentrations of hydrogen ions in a solution.
Strength of an Acid or Base
Strength of an acid or base depends on the number of H + ions or OH- ions produced by them respectively. Larger the number of H + ions produced by an acid, stronger is the acid. Similarly, larger the number of OH – ions produced by a base, stronger is the base.
The pH Scale
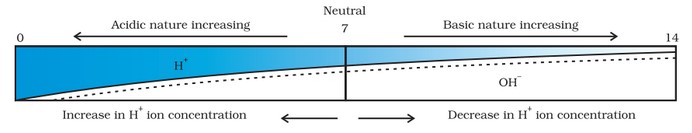
It is a scale used for measuring hydrogen ion concentration. The p in pH stands for potenz which means power in German. It has values ranging from 0 (very acidic) to 14 (very alkaline). pH is a number which indicates the acidic or basic nature of a solution.
Higher the hydronium ion concentration present in the solution, lower is its pH value [pH means power of hydrogen ions].
- If pH > 7, the solution is basic. • If pH < 7, the solution is acidic.
- If pH = 7, the solution is neutral.
Pure water is neutral because of the absence of free ions. A paper impregnated with the universal indicator is used for measuring pH. Now-a-days, a pH meter, an electronic device, is used to measure the pH value.
Importance of pH in Everyday Life
Following are the examples showing importance of pH in everyday life:
Plants and Animals are pH Sensitive
Living organisms can survive only in a narrow range of pH change, our body works normally within a pH range of 7.0 to 7.8.
When pH of rain water goes below 5.6, it is called acid rain. When acid rain flows into the rivers, it lowers the pH of the river water and makes survival of aquatic life difficult.
pH of the Soil
Every type of plant requires a specific pH range for healthy growth. Therefore, the nature of soil is known first by testing its pH and then a particular crop is grown in it.
It is also suitable for selecting the fertiliser for a particular crop by knowing the pH of the soil.
pH in Our Digestive System
HCl present in the stomach helps in the digestion of food. During indigestion the stomach produces too much acid, it causes pain and irritation. To correct the disturbed pH range, milk of magnesia (a mild base) is used as a medicine, which is also called antacid as it reduces the effect of acid (or acidity).
pH Change Leads to Tooth Decay
Tooth enamel is made up of calcium phosphate and is the hardest substance in the body. If the pH inside the mouth decreases below 5.5 (acidic), the decay of tooth enamel begins. The bacteria present in the mouth degrades the sugar and leftover food particles and produce acids that remain in the mouth after eating.
The best way to prevent this is to clean the mouth after eating food. To prevent tooth decay, toothpastes (basic) are used which neutralise the excess acid.
Self Defence by Animals and Plants through Chemical Warfare
When insects like honeybee, ant etc., bite, they inject an acid into the skin, that causes pain and irritation. If a mild base like baking soda is applied in the affected area, it gives relief.
pH in Plants
Stinging hair of nettle leaves injects methanoic acid in the skin which causes burning pain.
It is cured by rubbing the affected area with the leaves of dock plant, which often grows beside the nettle plant.
Salts
Salts are produced by the neutralisation reaction between acid and base. Salts of strong acid and strong bases are neutral with a pH value of 7. Salts of a strong acid and weak base are acidic with pH value less than 7. Salts of strong base and weak acid are basic in nature with pH value more than 7. Now, we will study about preparation and properties of some salts.
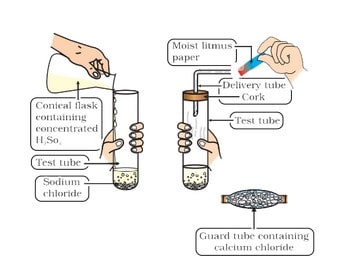
Common Salt [Sodium Chloride (NaCI)
Common salt is formed by the combination of hydrochloric acid and sodium hydroxide solution. It is the salt that we use in food. Sea water contains many salts dissolved in it.
It is obtained on a large scale from sea water by separating other salts from it. It may also be obtained from rock salt.
Deposits of solid salt are also found in several parts of the world. These large crystals are often brown due to impurities. This is called rock salt. Beds of rock salt were formed when seas of bygone ages dried up. Rock salt is mined like coal.
Common salt is an important raw material for various materials of daily use, like sodium hydroxide, baking soda, washing soda, bleaching powder etc.
Bleaching Powder [Calcium Oxychloride (CaOCl₂ )]
It is produced by the action of chlorine on dry slaked lime. It is represented as CaOCl₂, though the actual composition is quite complex.
On standing for a longer time, it undergoes auto-oxidation due to which bleaching action decreases.
Uses of Bleaching Powder
(i) It is used for bleaching cotton and linen in textile industry, for bleaching wood pulp in paper
industry and for bleaching washed clothes in laundry.
(ii) It is also used as a disinfectant for water to make it free of germs.
(iii) It is used as an oxidising agent in many chemical industries.
Caustic Soda [Sodium Hydroxide (NaOH)]
When electricity is passed through an aqueous solution of sodium chloride (called brine), it decomposes to form sodium hydroxide. This process is called chlor-alkali process because of the products formed, chlor for chlorine and alkali for sodium hydroxide.

In this process, chlorine gas is given off at the anode and hydrogen gas at the cathode and sodium hydroxide solution is formed near the cathode. The following three products produced in this process are very useful.
Baking Soda [Sodium Hydrogen Carbonate or Sodium Bicarbonate (NaHOD₃ )]
The soda commonly used in the kitchen for making tasty crispy pakoras is baking soda. It is a mild non-corrosive base. It is the major constituent of baking powder.
Sometimes, it is added for fast cooking. Chemically, it is sodium hydrogen carbonate. It is produced by using sodium chloride as one of the raw materials.
Manufacture of baking soda is shown in reaction given below:
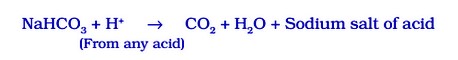
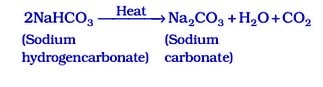
The following reaction takes place when it is heated during cooking :
Uses of Baking Soda
(i) For making baking powder, which is a mixture of baking soda (sodium hydrogen carbonate) and a mild edible acid such as tartaric acid. When baking powder is heated or mixed in water, the following reaction takes place:
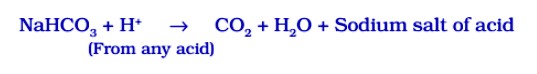
Carbon dioxide produced during the reaction causes bread or cake to rise making them soft and spongy.
(ii) Sodium hydrogen carbonate is also an ingredient of antacids. Being alkaline, it neutralises excess acid in the stomach and provides relief.
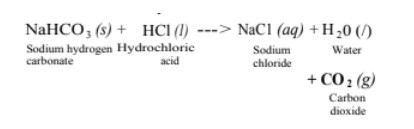
(iii) It is also used in soda-acid fire extinguishers.

When gypsum is heated above 400 K, dead burnt plaster (anhydrous CaSO4 ) is obtained which does not have the property of hardening. Plaster of Paris is a white powder and on mixing with water, it changes to gypsum giving a hard solid mass.

Uses of Plaster of Paris
(i) It is used by doctors for joining the fractured bones at the right position, for making plaster to support fractured bones.
(ii) It is also used for making decorative pieces and for making designs on ceilings.
Water of Crystallisation
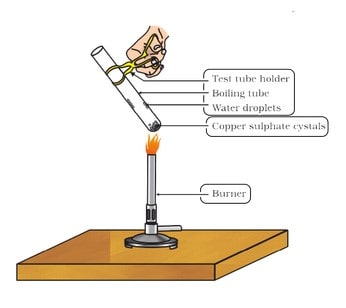
Crystals of some compounds seem to be dry (or anhydrous) but actually contain some water molecules attached to them. This water is called water of crystallisation and such salts are called hydrated salts. Water of crystallisation is the fixed number of water molecules present in one formula unit of a salt.
Five water molecules are present in one formula unit of copper sulphate (blue vitriol; CuSO4 . 5H ,0). Another salt which possesses water of crystallisation is gypsum (CaSO4 .2H20). It has two water molecules as water of crystallisation. This water is removed by heating the crystals of the hydrated salt. Plaster of Paris possesses 1/2 molecules of water of crystallisation.
NCERT Notes for Class 10 Science Chapter 2 Acids, Bases and Salts
CBSE Class 10 Science notes will assist students in studying the topic thoroughly and clearly.
These CBSE Class 10 Science notes were written by subject experts who made the study material very basic, both in terms of language and format.